Abridged Article: Lake Whatcom’s Low Dissolved Oxygen (DO) How Did It Get That Way And What We Can Do To Fix It
Editor’s Note: The following is an abridgement of the full scientific article that can be found on the Whatcom Watch website.

The above photo depicts Lake Whatcom on a late summer evening at sunset in Sudden Valley. photo: EJ Ledet
by EJ Ledet and Richard Bauman
What a beautiful lake and great place to live. We who live around Lake Whatcom, along with the city of Bellingham, Whatcom County and our friends, neighbors, and nearby residents, enjoy this wonderful source of great beauty and drinking water. We all, together, are responsible for the lake’s health and must do whatever is needed to preserve and improve its water quality.
Human activity on and around the lake, however, have caused stress — of particular concern is the lake’s declining amount of dissolved oxygen, which is crucial to its health. As the amount of dissolved oxygen declines, so does the lake’s water quality. The Environmental Protection Agency has designated the lake as “impaired,” and we propose both problem descriptions and solutions to elevate the lake’s water quality to remove that designation.
Solutions to that problem require understanding how oxygen enters the water and what causes its depletion. We two authors, scientists both, have studied this problem and have offered solutions to city officials. Neither of us has a financial stake in any decision.
This is a complex issue of multiple factors. Some physical conditions increase oxygen solubility, which means dissolved oxygen reaches all depths of the lake — a good thing. But there are other contributors that change that solubility or even minimize or prevent it. There are also biological and chemical causes which decrease, deplete or consume dissolved oxygen and biological and biochemical causes in the lake sediment which cause the lake’s health, i.e. water quality, to worsen.
We need to understand all these causes to be able to analyze cause and effect relationships and identify, develop and implement solutions to improve and maintain Lake Whatcom water quality.
We use a problem solving, Causal Analysis technique to define the problems, analyze their cause and effects and develop, fact-based cost-effective solutions.
Past Western Washington University (WWU) and Washington Department of Ecology (DOE) water studies used data on phosphorous, algae/chlorophyll and low dissolved oxygen in Lake Whatcom to drive regulations to manage phosphorus that also increased property taxes and assessed fees in newly created storm water districts. A DOE statement: “Researchers have determined that excess phosphorous in the lake is the main cause of declining oxygen levels. This study quantifies how much phosphorous the lake can process naturally and still supply enough oxygen to meet state standards.”
The Environmental Protection Agency recommends using Causal Analysis Diagnosis Decision Information System, or CADDIS, to help scientists and engineers in the regions, states, and tribes conduct causal assessments in aquatic systems. The system basically focuses on where a problem occurs vs where it does not occur, or when a problem occurs vs. when it does not occur, etc.
Supply Causes of Low Dissolved Oxygen
Warm lake water temperatures are caused by solar radiation intensity. Longer summer days increase the exposure, usually causing a decrease in oxygen solubility and concentrations of dissolved oxygen. Large and deep lakes like Lake Whatcom have layers of water, called strata, each with different properties usually defined by water temperature and density.
This is how it works.
Thermal stratification is usually seasonal with clear delineations between layers (the scientific names are: upper — epilimnion, middle — metalimnion, bottom-hypolimnion) during the summer. During warmer weather months, heat transfer from the sun through conduction, convection, and radiation changes the temperature and water density in the middle stratum which, in turn, causes one or more barriers or thermoclines to form. These barriers of temperature and water density prevent a natural upwelling and exchange between the strata and prevent reaeration/re-oxygenation and transfer of dissolved oxygen to the lower stratum.
Such problems tend to disappear in winter, when colder water temperatures and wind foster mixing of the levels.
The major source of dissolved oxygen in Lake Whatcom the colder months of winter is the exchange between air and water (i.e., Aeration/Oxygenation) and early spring mixing caused by wind, current, convection/conduction and turbulence.
A Whatcom Watch article published in June 2016, “Lake Whatcom Update: Decline of Water Quality Accelerates,” by April Markiewicz, lists several 2015 anomalies:
“The abnormal results they measured in the lake last year were probably caused by two factors: 1) the warm weather from February through July 2015, which raised surface water temperatures throughout the lake to be higher than any measured in the past 30 years, and 2) the water in Basin 3 did not completely mix from top to bottom during the winter months for the first time ever recorded, probably due to weak winter storms and the shape of the basin. This caused oxygen deficits in the bottom waters from 2014 to remain and get worse throughout 2015.”
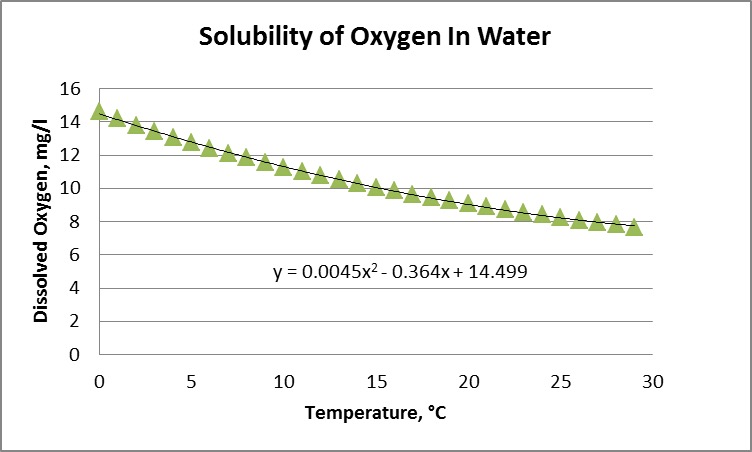
Warmer water temperature causes lower DO solubility/concentration/availability (see chart on previous page) shows the relationship of oxygen solubility and water temperature. Colder water yields higher levels of dissolved oxygen.
Solubility is affected by temperature, pressure, and salinity. Lake Whatcom is a fresh water lake, so salinity isn’t a factor. Atmospheric pressure is of marginal impact due to the lake’s slight altitude of 310 feet above sea level, which leaves water temperature as the key factor determining dissolved oxygen levels.
The article added:
“The mild winter plus warmer spring and early summer weather, caused the lake to thermally stratify into separate layers sooner with warmer, less dense, oxygenated water in the upper layers above colder, denser water below. Biological processes in the isolated deeper water quickly consumed the dissolved oxygen, creating unoxygenated water in Basins 1 and 2 and oxygen deficits in Basin 3.”
And the longer such stratification in summer, the shorter the atmospheric exchange in winter and lower dissolved oxygen levels deep in the lake. Longer periods of thermal stratification during warmer months can cause shorter atmospheric exchanges in colder winter months. This, in turn, causes even lower initial dissolved oxygen concentrations in the lower/deeper strata.
Other Factors
Biological and chemical processes consume dissolved oxygen in the deep strata. As the dissolved oxygen concentration is consumed in the water column, anaerobic bacteria, present in the sediment, begin to decompose other matter. Nitrates and nitrites are converted to ammonia. Sulfate salt, like iron sulfate, is converted to iron sulfide and hydrogen sulfide. Insoluble ferric phosphate is converted to soluble ferrous phosphate. All these by products from sediment anaerobic decomposition can be released into the above water column, decreasing the overall health and water quality of the lake.
Demand Causes for Low Dissolved Oxygen
Blue green algae are the typically named villains in depleting oxygen in the lake. They feed on the phosphates and nitrates in runoff that empties in the lake. Algal respiration consumes dissolved oxygen at night, and bacteria and microbes consume dissolved oxygen when algae die and decompose.
But algae are not alone. Other organic matter carried by storm water runoff, streams, creeks, and Lake Whatcom historical use, including saw mill debris, sunken logs, coal mine debris, leaf litter, decomposing wooden train trestles and remnants of sunken boats deposited in lake bottom sediment are factors. Bacteria and microbes aid in the decomposition of these and use/absorb dissolved oxygen through their digestion processes.

Pumping system proposal for increasing dissolved oxygen in Basins 1 and 2. Courtesy: Mitchell et. al. 2010
Solutions Development
Effective solutions address the majority of causes and prevent, change, mitigate the primary problem from recurring. Here are the basic formulae:
From-Current Lake: Decreased DO Supply + Increased DO Demand = Anaerobic (- DO) = BAD
To-Improve Lake: Increased DO Supply + Decreased DO Demand = Aerobic (+DO) = GOOD
Solution 1.
Oxygenation of the Lowest Stratum
Lake Whatcom contains three basins (see above graphic), numbered west to east. Basin 3 is the deepest, containing 96 percent of the lake’s volume. Its volume and depth also acts as a heat sink, which means that pumping water from its lowest stratum into the two shallower basins would generate the cold-water effects of the winter months. The costs associated with implementing and managing such a pumping system will have to be determined, but this solution could increase dissolved oxygen levels, which could prompt the Environmental Protection Agency to remove the 303 Impaired Body of water classification.
Solution 2.
Clean Flo’s Laminar Flow Inversion and Oxygenation
The most important part of CLEAN-FLO’s unique water improvement process is called “Continuous Laminar Flow Inversion and Oxygenation.” CLEAN-FLO systems are designed to completely mix the surrounding waters and evenly distribute dissolved oxygen throughout the sediments for efficient microbial utilization. Continuous laminar flow inversion oxygenates the water and removes toxic gases. CLEAN-FLO process oxygenates an entire body of water from bottom to top (inversion).
Clean Flo Diffuser creates tiny air bubbles. Set of diffusers oxygenate an entire body of water.
Laminar flow created by CLEAN-FLO systems is non-turbulent and will not increase suspended solids or increase turbidity. In fact, the opposite is true, suspended solids and turbidity will be reduced. CLEAN-FLO diffusers are placed on the bottom and are not suspended above the sediments. The diffuser releases bubbles from which oxygen is absorbed in the water, and which gently rise to the surface and foster more oxygen absorption. This oxygenation helps purge the water of carbon dioxide, which produces an environment that promotes better water quality. Other gasses such as hydrogen sulfide and ammonia are also purged from the sediments.

Clean Flo diffuser which creates thousands of air bubbles at the bottom of the lake to re-oxygenate and de-stratify the lake. Courtesy: Clean Flo International
Laminar flow inversion and oxygenation carries oxygenated, toxic gas-free surface water down to the bottom where it initially binds phosphorus and nitrogen to the sediments and reduces phosphorus, and nitrogen via biochemical pathways to re-establish the food chain.
Oxygenation enables beneficial microorganisms to feed on bottom non-living organic sediment. It enables aquatic insects to feed on the microorganisms, and fish to inhabit the bottom waters and feed on the insects, providing a valuable natural food source to improve fish growth and health.

This ring is caused by Clean Flo, a system to solve the dissolved oxygen problem by completely mixing the surrounding waters and evenly distribute dissolved oxygen throughout the sediments. Courtesy: Clean Flo International
We asked Clean Flo for cost estimates of the Clean Flo System and proposed evaluating this solution on a Pilot basis. Attached are the estimates from Clean Flo. We would also propose installing/testing the pilot in Basin 2 first and then decide on forward strategy basis typical water quality results which could be performed by the WWU laboratory and/or other qualified independent testing laboratory.
Basin 2 Pilot ($850,000-$1,000,000 first year and $200,000 each year to operate/maintain after installation) This estimate includes all set-up costs of $650,000 not including site work, electrical and building to house compressor. Annual maintenance, including testing costs among others, is estimated at $150,000 to $200,000. Installation time, depending on time of year and weather, to be two or three weeks. Evidence of improvement to be apparent in 30 to 60 days with continued improvement up to one year. Basin 1 $820,000-$1,000,000 first year and $200,000 to operate and maintain each year after installation) This estimate includes setup costs of $620,000 not including site work, electrical and building to house compressor. Other cost estimates were the same as those in Basin 2.
Both of these pilot programs aim to change lower water strata from anaerobic (oxygen deficient) to aerobic (oxygen rich) to nurture more nutrient metabolism. Strengthening the food chain results in larger, healthier fish populations which attract predators such as eagles, pelicans etc. These systems will also reduce toxic cyanobacteria, which produce toxins when subjected to algaecide treatment in the water purification process, and will reduce chemical costs in water treatment. They will also reduce the amount of E. Coli and other bacteria that create hazardous conditions.
Solution 3.
TMDL P (Total Mass Daily Load on Phosphorous)
The estimated price tag of this system is $100+ million dollars and a 50-year period to bring dissolved oxygen concentration in Basins 1 and 2 to 2002-3 levels.
“Spend $50 million in first 5 years, $10 million dollars per year to improve water quality in Lake is worth it.” says Dr. Matthews.
TMDL P Modeling assumes that main cause for dissolved oxygen depletion in Lake Whatcom is phosphorus which, in turn, causes Algae dissolved oxygen consumption via respiration and depletion via death and decay of aerobic bacteria and microbes. Although phosphorus contributes to organic and inorganic matter which is decomposed by biological, biochemical and chemical processes deposited on and in sediment, it represents a fraction of the total organic and inorganic matter composed of carbon, hydrogen, oxygen, nitrogen, sulfur, phosphorus and other minor elements. Constructing natural filtration systems will not address the phosphorus and other nutrients which are already present in the lake’s water and sediments.
We have raised concerns that the TMDL P solution will not address all causes of Low DO in Basins 1 and 2 and will not improve deep strata dissolved oxygen by itself.
Proposed Solution Scenarios to Implement the Most Cost-Effective Solutions to address Low DO Causes
We suggest implementation of the Clean Flo Pilot in Basin 2 in 2018 with future evaluations of its success to determine whether to implement that system in Basin 1 and eventually in Basin 3.
In summary, we disagree with the idea that phosphorus, and algae as its benefactor, is the primary cause of low oxygen levels in Basins 1 and 2. Although minimizing phosphorus levels in the Lake Whatcom watershed via (TMDL P) will help reduce or eliminate algae and minimize consumption of some of the low dissolved oxygen in Basins 1 and 2 lower strata, it will not address all causes of low dissolved oxygen depletion or address phosphorus and other nutrients already present in the lake.
We believe re-oxygenation of the hypolimnion via implementation of the most cost -effective reoxygenation/aeration solution coupled with a more cost effective TMDL stream and storm water management abatement plan is critical to restoring and improving oxygen concentrations in Basin 1 and 2.
Clean Flo Laminar Flow Aeration System
This solution will increase dissolved oxygen supply concentration and de-stratify the entire water column and effectively reduce chemical use in treatment costs, rebuild a healthy food chain sustaining and promoting fish growth and reproductive rates, and greatly reduces anaerobic biological and chemical reactions in the lake sediments and thus prevent the formation of hydrogen sulfide, ammonia, methyl mercury, iron, magnesium and lead.
It is the most cost-effective solution of the three proposed solutions for Lake Whatcom and will improve and enhance lake and drinking water quality and remove the EPA 303d impaired water body classification. Clean Flo will manage “how much phosphorous the lake can process naturally and still supply enough oxygen to meet state standards,” per the Dept. of Ecology’s TMDL P modeling study.
Clean Flo manages phosphorous and nitrogen inflow into the lake as well as phosphorous and nitrogen already present in the lake and eliminates the need to build/construct natural soil drainage filters.
We have written numerous emails and spoken with several city officials (CoB, WC, City Planners, WWU researchers, DOE regulators) and sent them a rough draft of the scientific article to found on the Watch website accompanying this article.
We heard back from Dr. Matthews (WWU) via email. She thought Clean Flo was more appropriate for Eutrophic Lakes and was working on writing her annual report for the city and could not meet with me to discuss my article because she was too busy at the moment. I did not hear back from Steve Hood with DOE. EJ met with one WC councilman and have scheduled to meet with another WC councilman in Jan 2018. EJ also received an email from the Director of Public Works that he would get back to him once he received feedback from his peers.
EJ spoke for three minutes to Whatcom County Council members at the Dec. 5, 2017, public meeting to discuss the newly formed Storm Water District Tax Assessment. EJ told members that we had concerns that the TMDL P would neither solve the lakes oxygen problem nor address the phosphorus and other chemicals already in the lake. EJ presented a low dissolved oxygen solution comparison table to show that re-aeration using the Clean Flo solution was the most cost-effective and would solve and improve water quality issues in Lake Whatcom.
One councilmen commented that aeration had been previously proposed and rejected, but that it should be revisited. The main issue with other aeration systems is disturbing sediment, high turbulence and mixing sediment in water column. Clean Flo’s laminar flow is non turbulent, and does not disturb or mix up sediment. In fact, it does just the opposite.
We continue to send Clean Flo updates to CoB, WC, WWU, DOE. We are placing articles in SV Views, Whatcom Watch Online, SMLLibety Road Website, and have remitted similar article for publication in Science Magazine and in WALPA. We do this freely as concerned citizens, researchers and taxpayers, because we want to add value to this beautiful area of the country.
End Notes
1. https://www.epa.gov/caddis; https://www.epa.gov/sites/production/files/2015-12/temp-cd_sim_1500.jpg ;
https://www.epa.gov/caddis-vol1/caddis-volume-1-stressor-identification-step-2-list-candidate-causes
2. Whatcom Watch Online “Lake Whatcom Update: Decline of Water Quality Accelerates”, Dr. Matthews et al. (2016)
3. Overview of Root Cause Analysis Techniques R. Bowen 5/18/2011
5. Minnesota Pollution Control Agency; Dissolved Oxygen TMDL-Protocols and Submittal Requirements, December 2008
6. Fondriest Environmental, Inc.: Water Temperature.” Fundamentals of Environmental Measurements. 7 Feb. 2014.
7. Identification of Fecal Escherichia coliform in Humans and Animals by Ribotyping, C.A. Carson, B. Shear, et al, Applied and Environmental Microbiology, December 2017,Vol 83, Issue 23
8. https://www.dropbox.com/s/2nr1rxu8u8j2ua2/Letter%2012-1-17.pdf?dl=0Clean Flo
9.https://www.dropbox.com/s/92gj04o237y9t0a/Lake%20Whatcom%20TMDL%202002%20Study%20.pdf?dl=0
_________________________________
E.J. (Enoch) Ledet has over 40 years experience as a chemist, biochemist, and causal analysis investigator/facilitator in the petrochemistry industry. He specialized in ensuring product integrity in both laboratory and field operations management and in the design and use of laboratory quality assurance systems.
Richard Bauman is a chemical engineer with 35 years experience working for Exxon- Mobil. He was the laboratory director responsible for research and development of gas conversion and shale retorting processes.